magnesium electron affinity|electron affinity equation : Tuguegarao The electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative .
Use the Symbol Viewer to find more symbols. The Mac doesn't have as many codes as a Windows computer, but you can find lots of different symbols in the Symbol Viewer: Click the Apple menu and select "System Preferences." Click the "Keyboard" option and then check "Show viewers for keyboard, emoji, and symbols in .
PH0 · trend for electron affinity
PH1 · how many electrons does magnesium have
PH2 · element with highest electron affinity
PH3 · electron affinity vs electronegativity
PH4 · electron affinity trend and exceptions
PH5 · electron affinity equation
PH6 · Iba pa
Good customer service is a must for a reputable casino and Fair Go Casino is one of them. The team helps 24/7 with live chat, phone, or email. You can also find fast answers in the FAQ on the official website. It reviews and solves many common issues without contacting support. Fair Go Aussie casino improves by getting feedback from players.Monstruos y bestias con proyectiles / Infantería de proyectiles monstruosa / wh3_main_ogr_inf_maneaters_3 Comehombres (pistola Ogra) Gracias a los espeluznantes trofeos de sus muchas batallas en tierras lejanas, es imposible que dos de estos mercenarios vayan armados de la misma manera.
magnesium electron affinity*******August 11, 2023. Periodic table with electron affinity values is shown above. The values of electron affinity are given in kJ/mol. Values in parentheses ( ) are predicted values. Electron affinity is the amount of .
Electron affinity of Magnesium is — kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is .
Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. The latter can be regarded as the ionization energy of the –1 ion or the zeroth ionization energy. Either convention can be used.
Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative .
Electron Affinity (kJ/mol) Electron Configuration: H: 72.8 : 1s 1: He <0 : 1s 2: Li: 59.8 [He] 2s 1: Be <0 [He] 2s 2: B: 27 [He] 2s 2 2p 1: C: 122.3 [He] 2s 2 2p 2: N <0 [He] 2s 2 2p 3: O : 141.1 [He] 2s 2 2p 4: F: 328.0 [He] 2s 2 . The electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative .magnesium electron affinity Example of IE 1 of Magnesium: Mg(g) -> Mg + (g) + e-I 1 = 738 kJ/mol. IE 1 stands for the first ionization energy: the energy the atom requires to expel the first .Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale) The tendency of an atom to .
The Kossel shell structure of magnesium. Atomic spectrum. A representation of the atomic spectrum of magnesium. Ionisation Energies and electron affinity. The electron affinity of magnesium is 0 kJ mol ‑1. .
The electron affinity ( Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to . Chlorine - Electron Affinity. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule when an electron is added to the atom to form a negative ion. . Magnesium is a shiny gray solid which bears a close physical resemblance to the other .The electronic affinity is amount of energy, that is released during the attachment of the electron to the neutral atom. As a result of such attachment, a negative ion (anion) is formed. Electron affinity is related to electronegativity of elements.Simply speaking, the greater the affinity of electrons, the more eagerly the atoms of a given element join .The electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. A fluorine atom in the gas phase, for example, gives off energy when .
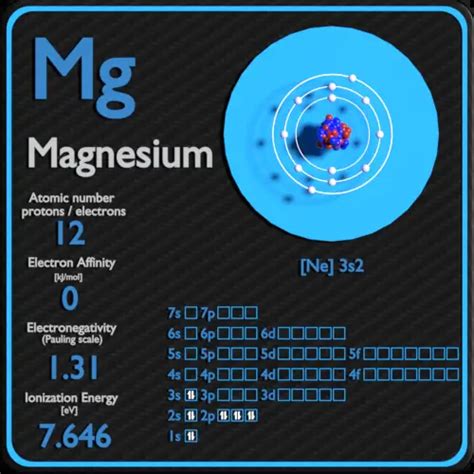
The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favourable the electron addition process is. Not all elements form stable negative ions in which case the electron affinity is zero or even positive.Magnesium is the eighth most abundant element in the Earth’s crust, but does not occur uncombined in nature. It is found in large deposits in minerals such as magnesite and dolomite. . Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale)The electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion. A fluorine atom in the gas phase, for example, gives off energy when it gains an electron to form a fluoride ion. F ( g) + e - F - ( g) Ho = -328.0 kJ/mol.Electron Affinity – Magnesium. Electron affinity of Magnesium is — kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 6 and 7 of the Periodic Table. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. This is more easily seen in symbol terms.Calculated Electron Affininty for MgO (magnesium oxide) Experimental Electron Affinity is 1.62 ± 0.025 eV Please note! These calculated electron affinity energies have the vibrational zero-point energy (zpe) included, but the zpe has NOT been scaled.Click on an entry for more details, including the ionization energy with a scaled zpe.

Electron Affinity. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. X + e – → X – + energy Affinity = – ∆H
electron affinity equation Electron Affinity. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. X + e – → X – + energy Affinity = – ∆H Sulfur - Electron Affinity. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule when an electron is added to the atom to form a negative ion. . Magnesium is a shiny gray solid which bears a close physical resemblance to the other .Summary. The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right corner of .
Electron affinity (E.A.) is the energy change that occurs when an electron is added to a gaseous atom. Electron affinity can further be defined as the enthalpy change that results from the addition of an electron to a .Extremely high negative electron affinity of diamond via magnesium .
Lithium - Electron Affinity. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: the change in energy (in kJ/mole) of a neutral atom or molecule when an electron is added to the atom to form a negative ion. . Magnesium is a shiny gray solid which bears a close physical resemblance to the other .We know that the so, um has a higher electron affinity than the potassium, and magnesium has a higher electron affinity, then the calcium. And so now we can use this to help goddess in terms of figuring out which species are greater have greater electron affinity than the others. And so one other important thing to note is stability. And so, if .
Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy change=}EA \label{7.5.1}[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to .Electron Affinity in the Periodic Table of Elements. Electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. Alternatively, it can also be defined as the energy required to detach an electron from the singly charged negative ion.
PST to CST converter. Quickly convert Pacific Standard Time (PST) to Central Standard Time (CST) accurately using our converter and conversion table.
magnesium electron affinity|electron affinity equation